Today in Biology Part 1 — Leqembi: An Overview of Alzheimer's Newest Drug
Published on July 19, 2023
It happened. Alzheimer’s disease, long thought of as an incurable dementia, finally has an FDA-approved treatment. As of January 2023, Biogen and Eisai’s ‘Leqembi (mentioned in passing in Episode 0)’ was granted accelerated approval, meaning that although human trials were yet to be completed, the FDA gave Leqembi approval to be used in treatment of Alzheimer’s. But as of July 6, 2023, this uncertain status is no more. Leqembi was granted traditional full approval by the FDA, after a lengthy approval process and much controversy. But let’s take a peek into the past, at the makings of Leqembi, and the history of Alzheimer’s disease.
Described first in 1906 as “A peculiar severe disease process of the cerebral cortex,” Alzheimer’s disease is now thought to comprise upwards of 60% of all dementia cases. Common symptoms include social/self neglect, mild/severe mood swings, general behavioral problems, and most infamously, forgetfulness of recent events. Although the cause of Alzheimer’s is yet a mystery (it is suspected to be genetic, but there are known to be many environmental factors as well), there has been much progress in discovering its pathology. The most likely theory (for now) is called the Amyloid Cascade Hypothesis,
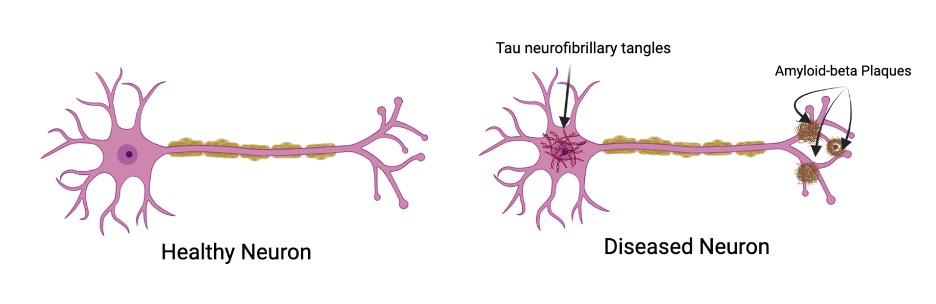
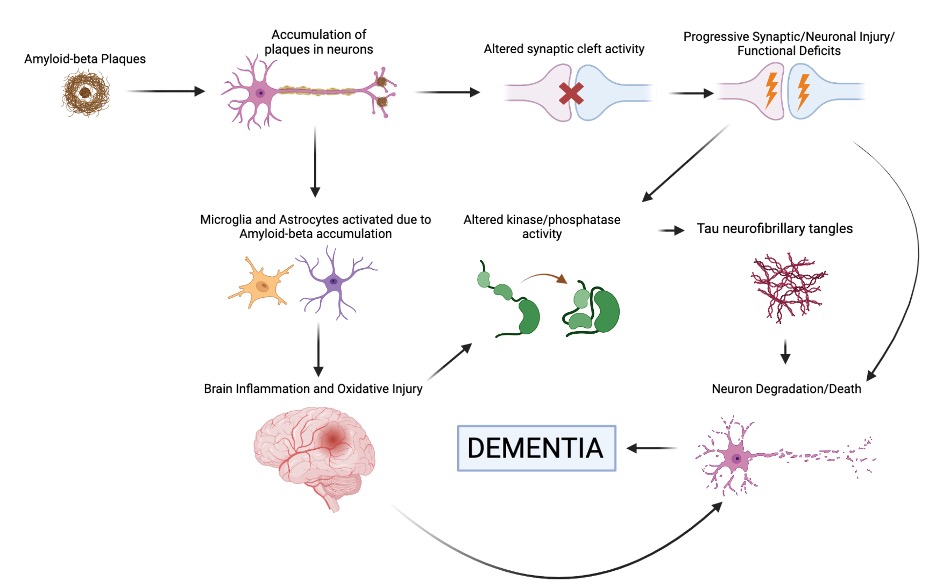
Here on the left we have a standard neuron, happy and healthy. Over on the right, we have a neuron representative of Alzheimer’s disease: the accumulation of substances called Tau neurofibrillary tangles(typically found in the cell body) and Amyloid-beta plaques (typically found at axonal endings) affects neuronal connectivity and function, thus leading to the onset of symptoms found in Alzheimer’s disease. The tau protein tangles are hypothesized to be the result of disease progression, due to oxidative stress that leads to chronic inflammation (a known characteristic of many neurodegenerative diseases), which in turn leads to the DNA and cell damage found in Alzheimer’s cells. Here’s a flowchart to help visualize and detail the process more:
To treat the resulting dementia, there has been much research into various drugs (mostly cholinesterase inhibitors, as acetylcholine is known to increase communications between nerve cells), but nothing disease-modifying was FDA-approved until June 2021, when Biogen’s aducanumab (Aduhelm) was given accelerated approval. However, this approval was not without controversy: the merits of the drugs were initially rejected in November 2020 by an FDA Advisory Committee, as they stated that the drug had questionable efficacy and the studies failed to show strong enough evidence to be approved. However, against this judgment, Aduhelm was approved, leading to the resignation of three panelists who were against its approval as a means to protest. In addition, drug administration was expected to cost $56000 dollars annually, until an eventual price slash to $28000. The drug was eventually discovered to have several adverse effects, namely Amyloid-related imaging abnormalities (ARIAs). These included edema in the brain, microhemorrhaging, superficial siderosis, causing symptoms such as headaches, vomiting, seizures, confusion, and decrease in level of consciousness. As a result of the high cost, minimal benefits (if any), and significant side effects, Biogen all but abandoned Aduhelm by nixing its funding for marketing and development, and having its CEO (Michel Vounatsos) step down. Dubbed by Aaron Kesselheim (one of the panelists who left Biogen) as “probably the worst drug approval decision in recent US history,” the story of Aduhelm came to an effective end in April 2022.
With Aduhelm’s failure, there was still no disease-modifying medicine for Alzheimer’s disease. Biogen decided to try again, this time partnering with the Japanese company Eisai to explore lecanemab (Leqembi), with the FDA accepting an application for the drug in July 2022. Similar to Aduhelm, Leqembi targeted Amyloid-beta plaques, and was discovered to be significantly effective in reducing plaque accumulation. Like Aduhelm, Lequembi was also granted accelerated approval by the FDA in January 2023, despite data pointing to the same side effects that were present with aducanumab administration being present with lecanemab. In addition, the pricing is extremely similar as well, with Leqembi being priced at $26500 to Aduhelm’s $28000. So what even is the difference, and is it worth caring about? In terms of mechanism, Leqembi attacks Amyloid-beta plaque accumulation at an earlier stage than Aduhelm; here is a figure showing the stages of Amyloid-beta plaque development:
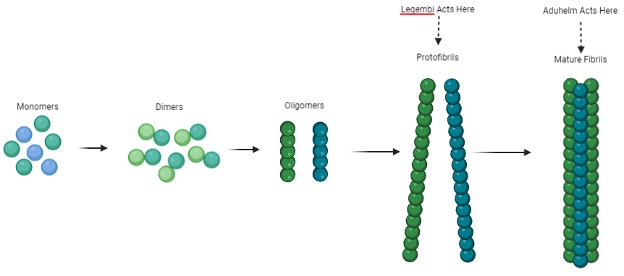
As far as the trials, there was much more rigorous testing with Leqembi than with Aduhelm, and it was discovered that Leqembi was slightly less likely to cause severe side effects. Rather than being administered via spinal infusion like Aduhelm, Leqembi is given intravenously, with clinical trials demonstrating a 27% prevention rate of functional deterioration. However, one of the biggest and most important changes from Aduhelm is the price. While we mentioned previously that the prices between the two were very similar, that only speaks for the market price; Leqembi is covered by Medicare, an advantage its predecessor did not have the privilege of, with estimates showing that patients will not have to pay over $7000 out-of-pocket. Leqembi is by no means a perfect cure, but it provides us great insight into what all has yet to be done. As more trials are performed and additional research is conducted, we will hopefully find a way to minimize the side effects and increase the effectiveness of Alzheimer’s disease treatments. It’ll take lots of time and money but for now, however, let us celebrate this monumental footstep towards a bright future, and hope for the best coming forward. Thank you all for reading, and stay curious!
Signing Off,
Parth and Chinmay